There are several validation rules set forth by the DEA that must be met when electronically prescribing controlled substances. These rules are as follows:
Schedule II Controlled Substance Prescriptions Rules:
- All Schedule II electronic prescriptions must be signed by the prescribing clinician using two-factor authentication. This is done using either a mobile two-factor authentication or USB Token two-factor authentication within the application.
- The prescriber's DEA Number is required.
- Both the prescriber and pharmacy must be registered with the Controlled Substance service level within the Surescripts directory.
- No Refills are allowed.
- To prescribe more than 1 fill, use the Effective Date field to send a prescription valid for a future date in place of a refill.
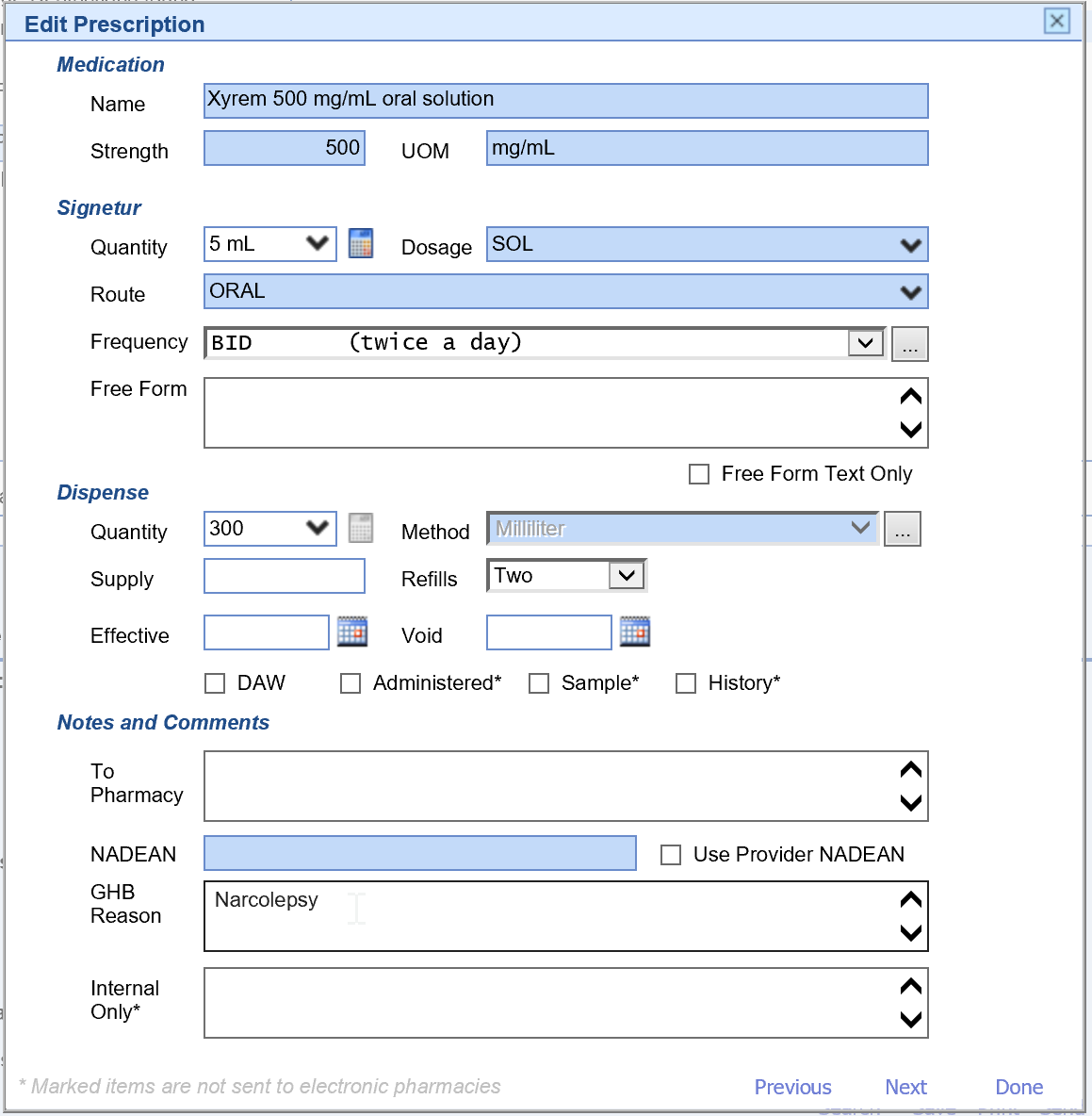
- When prescribing Gamma-Hydroxyburic acid/GHB/Xyrem, it is required to add a note for the medical need in the GHB Reason field.
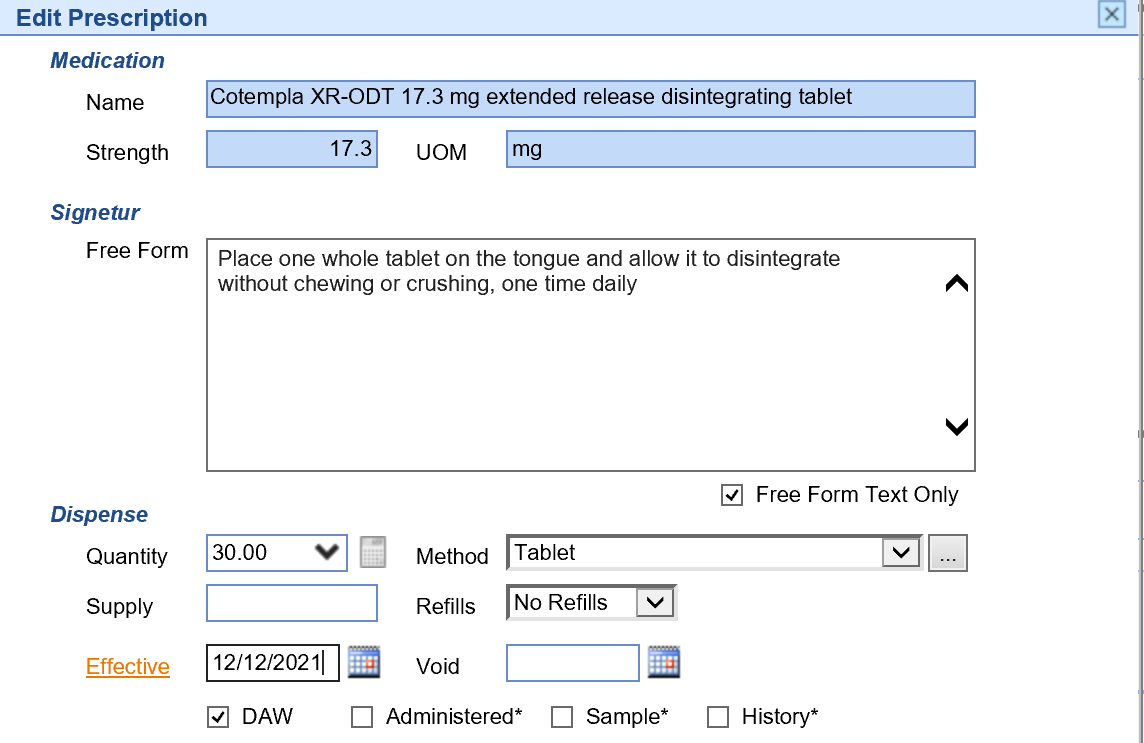
Schedule III-IV Controlled Substance Prescriptions Rules:
- All Schedule III-IV electronic prescriptions must be signed by the prescribing clinician using two-factor authentication.
- The prescriber's DEA Number is required.
- Both the prescriber and pharmacy must be registered with the Controlled Substance service level within the Surescripts directory.
- A maximum of 5 Refills (6 total fills) can be prescribed.
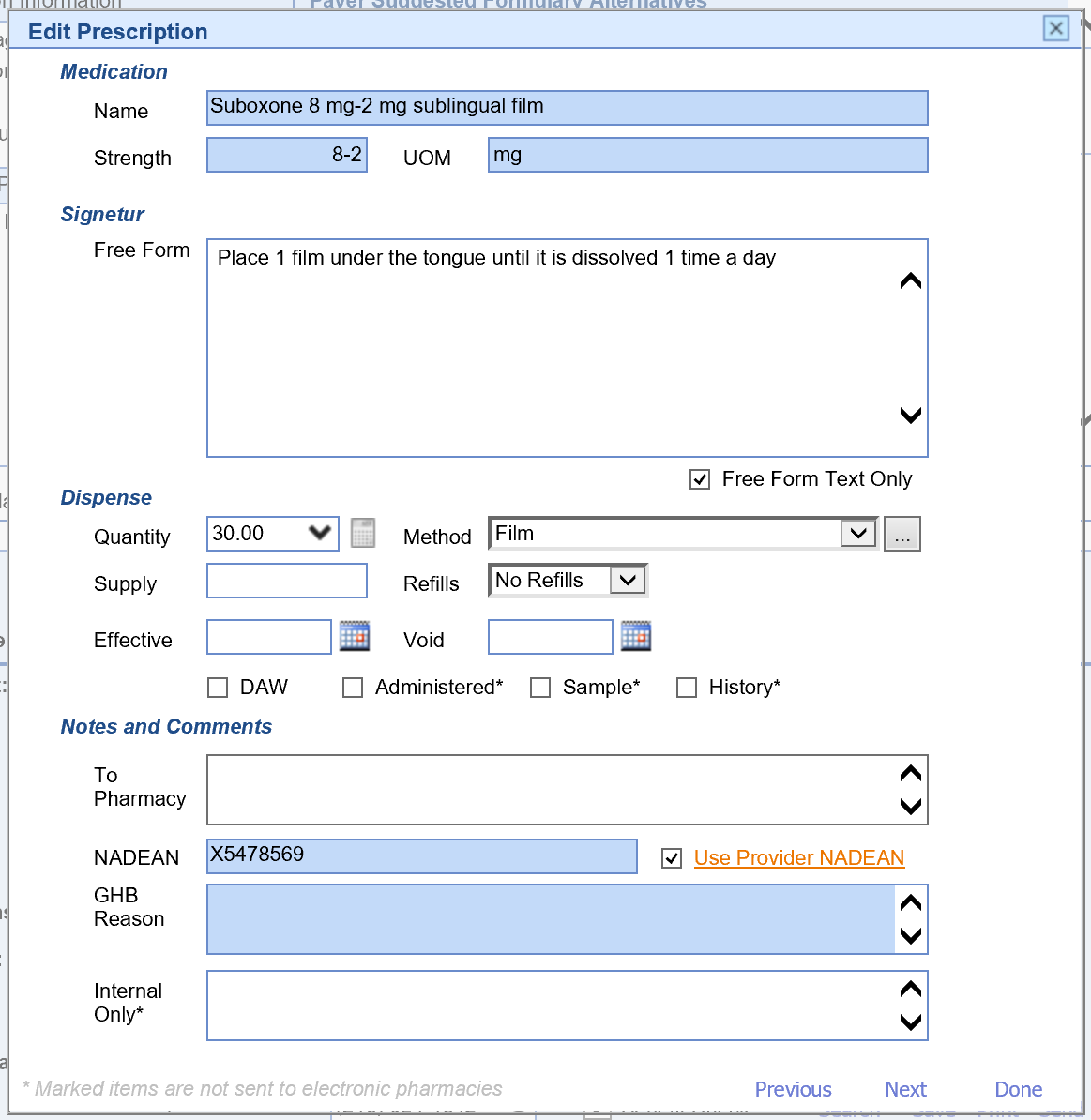
- Most electronic prescriptions for buprenorphine also require the prescriber's NADEAN number. Select the Use Provider NADEAN checkbox to send your NADEAN with the EPCS prescription.